Lynn Rothschild
NASA Ames Research Center
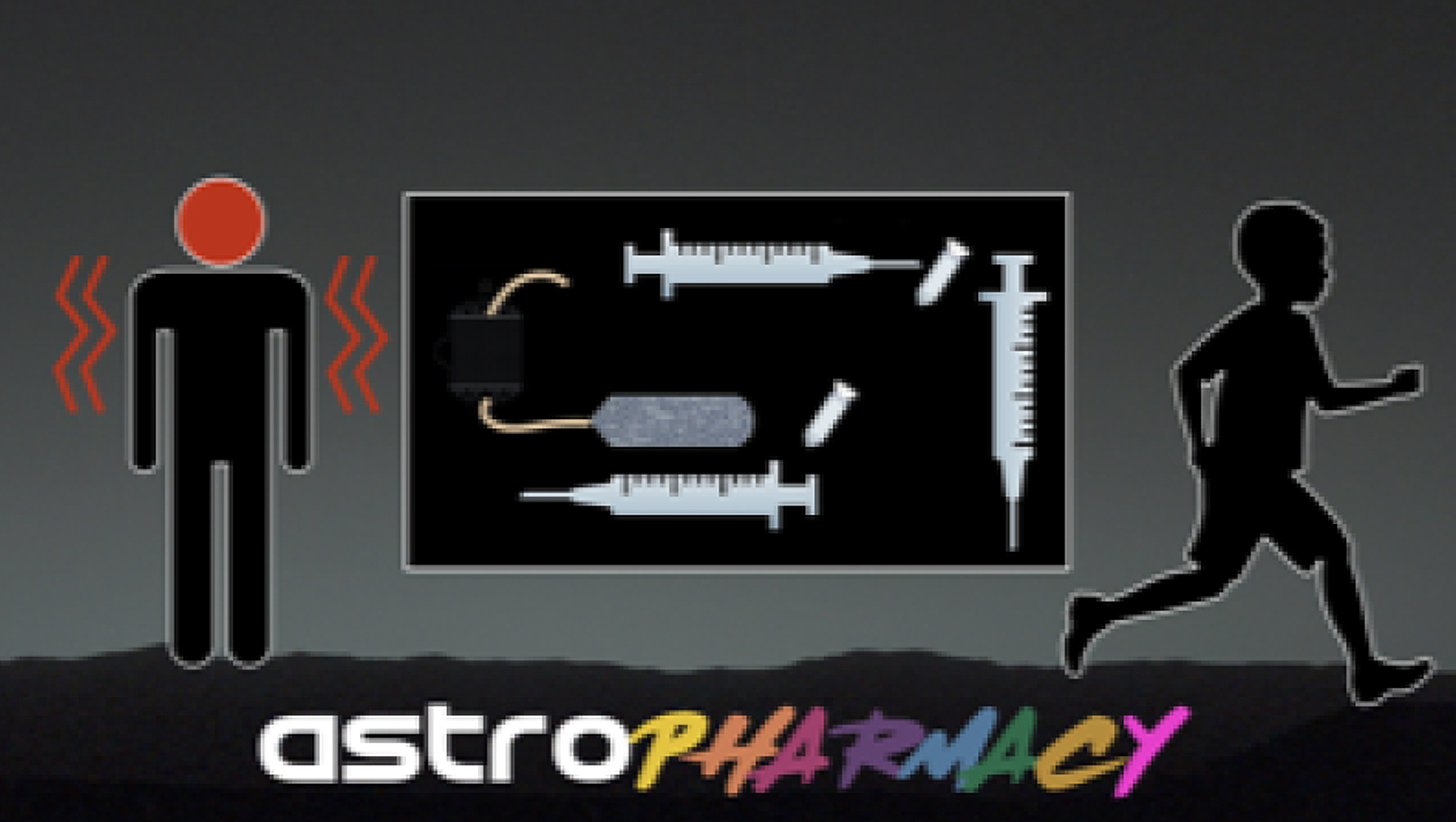
Disease is an inherent part of being alive, and thus disease prevention, diagnosis and treatment will be critical to human deep space missions. Pharmaceuticals are used to diagnose, treat, cure or prevent disease, but suffer from lack of stability on Earth and even more so in the space environment. What if small quantities of pharmaceuticals could be made in space, on site, on demand? An important class of therapeutics, the biopharmaceutical or ‘biologic’ (peptide or protein drugs) would be particularly amenable to in space manufacturing. Many of the medical conditions and emergencies that astronauts are known to – or could likely – face could be treated effectively with these agents. These protein-based drugs, approved by the FDA, are now used in the clinic to treat embolisms, hemorrhages, renal stone formation, bone loss, infection, thrombotic complications, etc. Unfortunately, biologics degrade in 6 months, even with refrigeration. We propose a concept to tailor-make drugs, initially non-glycosylated biologics, by pre-programming cells that are space hardy due to being in the spore form, to produce them with the addition of ~1 mL of sterile medium using a lightweight, small volume system adapted from standard laboratory protocols and enabled by judicious genetic engineering prior to launch. Ultimately, a more flexible system could be considered that eliminates the need for pre-programming cells where a dried cell-free transcription/translation (TxTl) system is used instead for production, but a similar purification protocol. This strategy could be extended to glycosylated biologics as well. It’s a concept we term an “Astropharmacy.” This on-demand approach removes concerns about pharmaceutical degradation due to time or radiation during space travel, and minimizes the resources needed to provide safe effective pharmaceuticals needed to keep crew members healthy.
The proposed work has four objectives:
- We will raise the TRL to 3 by (1) the synthesis and purification of biologics, (2) testing their purity and activity, and (3) quantifying parameters for production. We have chosen two drugs—G-CSF and Teriparatide
- Assess requirements for implementation in space (mass, etc.) in the context of a long-stay Mars mission
- Identify key knowledge gaps & outline roadmap for technology development
- Assess the impact of technology for terrestrial applications
If successful in developing the Astropharmacy technology, the quality of astronaut healthcare will be improved by eliminating concerns of biologic drug degradation. Finally, such systems could have spin-offs for on-demand, on-site production of small quantities of other useful peptides/proteins in space, as well as on Earth, an economical path forward for orphan drugs, and improved stability for prototyping genetic circuits in cell-free systems.